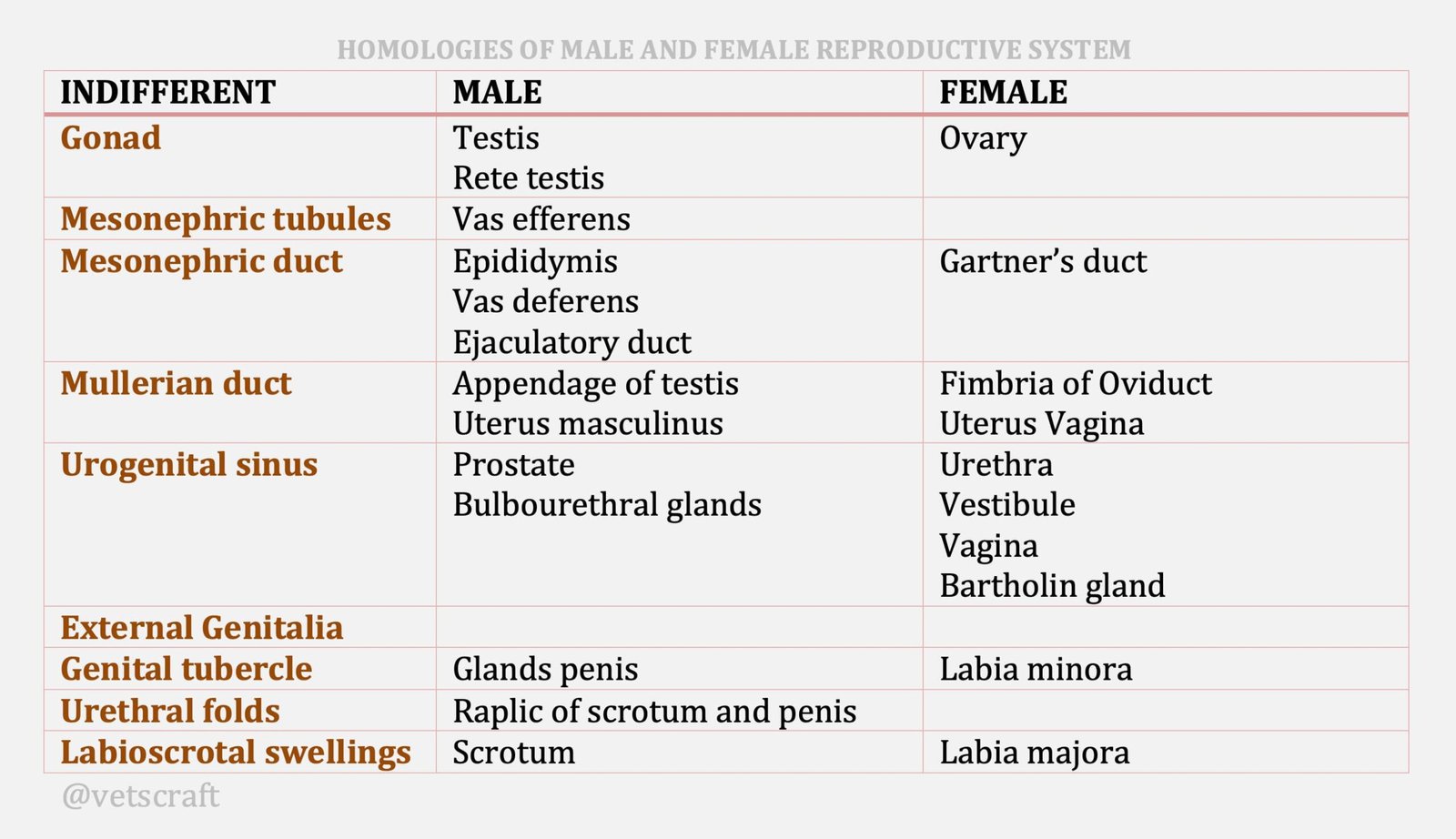
TABLE OF CONTENTS
Embryo Transfer Technology (ETT)
Embryo transfer technology is a technique by which embryos are collected from a donor female and are transferred to recipient females, which serve as surrogate mothers for the remainder of pregnancy.
History of Embryo Transfer
Embryo transfer (ET) was first performed and recorded by Walter Heape in 1890. He transferred two Angora rabbit embryos into a gestating Belgian doe. She went on to produce a mixed litter of Belgian and Angora bunnies.
In India the first ET project ‘Cattle Herd Improvement for Increased Productivity Using Embryo Transfer Technology’ was established in 1987. The first buffalo calf from frozen thawed embryo was born in 1991.
Advantages and disadvantages of an ET Program
Advantages
- Increase the number of offspring sired from superior females.
- Results in faster genetic progress.
- Increase the frequency of desired matings, capitalizing on excellence of a mating.
- Obtain offspring from old or injured animals incapable of breeding or calving naturally.
- Increased farm income through embryo sales.
- Exportation and/or importation of embryos is easier than with live animals.
Disadvantages
- Can be cost prohibitive and success rates are less than AI.
- Cost and maintenance of recipient females.
- Requires a technician with the skills to flush embryos from the reproductive tract.
- Possible spread of disease through recipients.
Process of Embryo Transfer
Process of embryo transfer technology involve following steps:
- Selection of the Donor
- Selection of the Recipient
- Superovulation
- Insemination of the Cow
- Flushing (Non-Surgical Embryo Recovery)
- Evaluation
- Freezing
- Transfer of Embryos
- Pregnancy Rates Resulting from Embryo Transfer

1. Selection of the Donor
Animal that donates embryos should have following features:
Selection criteria are used to select a donor is based on genetic merit, reproductive performance, progeny performance.
Embryo from a donor cow does not guarantee a superior calf. Each embryo has a different genotype and more than likely a different phenotypic expression due to its environment.
Reproductive soundness like:
- Animals must be exhibiting regular estrous cycles.
- At least 60 to 90 days post calving.
- Eliminate cows with a history of reproductive problems.
- A history of no more than two breedings per conception.
- Previous calves having been born at approximately 365day intervals.
- No parturition difficulties or reproductive irregularities.
She should be maintained at the level of nutrition appropriate for her size and level of milk production. Both the very obese cow and the thin cow will have reduced fertility, so it is important that the donor cow be in an appropriate body condition score at the time of embryo transfer.
2. Selection of the Recipient
Animal that receives embryo from donor:
- Recipients (also known as surrogate) are the greatest single cost of an ET program because they need to be at the same stage of the estrous cycle as the donor when she donated the embryos. Estrous synchronization is typically used to manipulate recipients so they are at the correct stage of the estrous cycle.
- Females should be healthy, good body condition, and vaccinated for all the common reproductive diseases.
- Females should have been through at least two normal oestrous cycles before use.
- Pregnancy rates are greatest when day of the estrous cycles of the donor and recipient are within 24 hours of each other. The recipient should exhibit estrus from 24 hours before to 12 hours after the donor was in estrus. Embryos are typically transferred on day 7 of the estrous cycle. Management components of ET Program.
3. Superovulation
Superovulation is the process of super stimulating the ovaries with FSH to produce multiple oocytes and it is the least predictable step of embryo production. There is tremendous variation in the number of embryos recovered after superovulation, which is due to variables like animal age, breed, lactation status, nutritional status, season, and stage of the estrous cycle when FSH treatment is initiated.
The process of superovulation includes frequent (2x daily) treatment of females with FSH for a period of four days. Treating with FSH allows for the recruitment, growth, and development of multiple ovarian follicles. Horses are typically not super-stimulated since they can only ovulate one follicle per ovary due to the presence of ovulation fossa.
Females are administered prostaglandin during the third day of FSH injections to regress any CL present. Females usually exhibit estrus within two days after the last day of FSH at which time they are artificially inseminated two times 12-24 hour apart.
Expected response is 5 to 12 embryos that are transferable and/or freezable. However, 0 to 20 oocytes can be ovulated, with nearly all being fertilized and transferable.
4. Insemination of the Cow
Twice AI with a 10-12 h interval beginning 4 -6 h after the onset of estrus, to cover the range of time over which the ovulations may occur.
Depending on the quality of the frozen semen, a double inseminating dose may be used at each insemination especially in cows with a large pendulous uterus.
5. Flushing (Non-Surgical Embryo Recovery)
Most embryos are collected by a non-surgical process between day 6 – 8 (estrus = day 0) of the estrous cycle with day 7 being the most common. Since embryos have not hatched from the zona pellucida yet, they are easier to locate with a microscope after being flushed from the uterus.
A specially designed instrument called a Foley catheter is used for the flushing procedure. The Foley is a 2-way catheter that has one channel for inflation of a balloon at the end of the catheter plus an additional channel for the inflow and outflow of flushing medium.
Flushing Process
The donor is administered an epidural anesthesia (lidocaine) to relax the rear leg muscles during the flushing process.
The Foley catheter is inserted through the vagina, cervix and into one of the uterine horns where the balloon is inflated. The inflated balloon will seal off the anterior portion of the uterine horn, which prevents fluid from leaking out of the uterus as flushing media is added to the uterine horn to recover the embryos. After a small amount (100-300 m L) of fluid is “flushed” into the uterus the technician recovers the fluid from the uterus. This process is repeated on the other horn in cattle. In horses, the entire uterus is flushed since uterine horns are minimal in size in the horse.
The flushing media recovered from the uterus is filtered (75 μm pore size) to assist in separating embryos from the flushing media. Once the embryos are recovered they are evaluated for stage of development and graded for quality.
6. Evaluation
Embryos can be maintained at room temperature for 12-24 hours at a pH of 7.1 to 7.5 when holding media is changed every few hours. Excessive temperature harms embryos (39o C). It is recommended that embryos be frozen within two hours after flushing.
Embryos are classified by stage of development and graded based on gross morphological appearance. It takes a great deal of experience to do this well.
Quality Grades or Scores
- Excellent (1): Embryos with few or no recognizable imperfections, such as poor compaction or variation in cell size.
- Fair (2): Embryos that show disarrangement, like a small embryonic mass with irregular shape or a large number of extruded and/or dead cells. Usually 1 to 2 days retarded development.
- Poor (3): Embryos with signs of cellular degeneration, like a small ICM and several extruded cells. Development has been retarded by more than 2 days.
- Dead or Degenerating (4): Embryos contain mostly dead cells and very few live. Tiny cell mass that is disorganized in appearance. Not worth transferring.
After classification, embryos are prepared for the next processing procedure, which may include sexing of embryonic cells and (or) splitting the embryo in half to make twins. Embryos are either transferred immediately into a recipient or frozen for storage.
7. Freezing
Cryoprotectants like glycerol and ethylene glycol are used to dehydrate cells and protect the cells of the embryo during the freezing and thawing process. Ethylene glycol is the cryoprotectant of choice in cattle since embryos can be frozen in a single step and eventually thawed in a single step and immediately transferred into a recipient. Embryos frozen in ethylene glycol are known as direct transfer (DT) embryos. Freezing embryos is a common practice in cattle but not in horses since their embryos do not freeze well.
Embryos are equilibrated for 5 to 10 minutes in cryoprotectant freezing medium.
Embryos are loaded and stored in a 0.25cc polyvinyl straw.
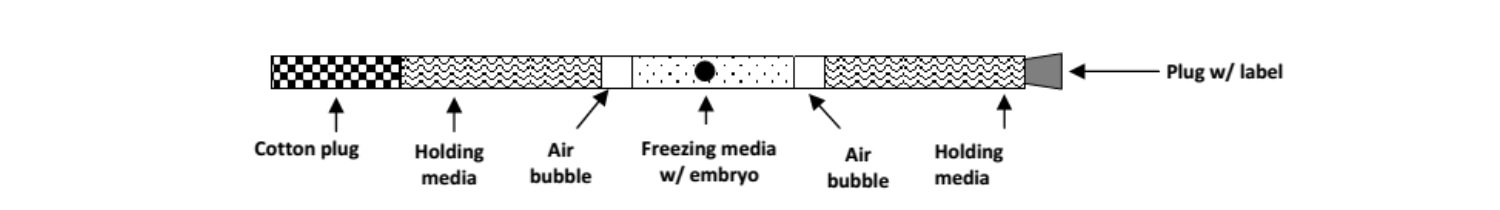
Embryos are frozen in a step-wise procedure in a special freezing machine:
- Embryo is placed in a freezing unit at -6°C for 5 minutes.
- Embryo is “seeded” with forceps cooled in liquid nitrogen to facilitate uniform freezing.
- Held at -6 to -7°C for another 5 minutes.
- Then cooled at 0.5°C/minute to -32°C.
- Hold at -32°C for 15 minutes and straws are plunged into liquid nitrogen (-196°C).
8. Transfer of Embryos
Transferring embryos can be transferred in two different manners:
(1) Same day transfer: Embryos are transferred into recipients on the same day they are collected from the donor. Transferring fresh embryos results in greater pregnancy rates, but requires a great deal of coordination to get recipients and donors on the same day of the estrous cycle when flushing occurs. More common in horse compared to cattle.
(2) Transfer of Frozen Embryos: Embryos are frozen the day they are flushed and then transferred at a later date.
Although pregnancy rates for frozen embryos are slightly less than fresh, the process has numerous advantages including:
- Frozen embryos offer important logistical and economic advantages. More cows can be flushed in a day, which facilitates freezing of embryos.
- Frozen embryos can be marketed and imported and/or exported more easily.
Process of Transferring Embryos
The recipient is palpated to determine the presence and location of the CL (right vs. left). Recipient is administered an epidural (lidocane) to relax the muscles in the pelvic area.
If the embryo is frozen it is thawed in a warm water bath (92°F) for < 30 sec and placed in a specially designed transfer gun and covered with a sterile sheath.
The transfer gun is passed through the vagina, cervix, and into the uterine horn on the side as the CL. The embryo is deposited 1/3 the way up the uterine horn. Air bubble Holding media Cotton plug Freezing media w/ embryo Holding media Plug w/ label Air bubble 6.
Pregnancy rates are greatest when the day of the estrous cycles of donor and recipient are within 24 hours. Recipients should be in heat 24 hours before to 12 hours after the donor was in estrus. The embryos are typically transferred on day 7 of the estrous cycle.
9. Pregnancy Rates Resulting from Embryo Transfer
When done by experienced technician, the transfer of fresh embryos yields pregnancy rates of 70 to 80%, while transferring frozen embryos yields pregnancy rates of 50 to 60%.
The average cost to produce an embryo derived calf is Rs.15000 to 20000. This does not include the cost of the recipient, which could be between Rs. 40 to 60 thousand/animal.