TABLE OF CONTENTS
Drug discovery and clinical trials
Drug discovery
Most new drugs or drug products are discovered or developed through one of the following methods:
- Identification or elucidation of a new drug target.
- Rational drug design based on an understanding of biological mechanisms, drug receptor structure, enzymes sites etc.
- Chemical modification of an existing molecule.
- Screening for biologic activity of natural products, previously discovered chemical entities, and libraries of peptides, nucleic acids, and other organic molecules.
- The explosive growth in biotechnology and cloning has paved the way to identify genes to produce peptides and proteins. Efforts continue to focus on the discovery of new targets and approaches, based on studies with genomics, proteomics, nucleic acids and molecular pharmacology for drug therapy.
- Combinations of known drugs to obtain additive or synergistic effects or a repositioning of a known drug for a new therapeutic use.
Drug screening
- Drug screening refers to a sequence of experiments and characterization using a variety of biologic assays at the molecular, cellular, organ system, and whole animal levels to define the activity and selectivity of the drug. For example, anti- infective drugs may be tested against a variety of infectious organisms some of which are resistant to standard agents.
- The molecule will also be studied for a broad array of other actions to establish the mechanism of action and selectivity of the drug. This will reveal any suspected and unsuspected toxic effects.
- Occasionally, an unexpected therapeutic action is discovered by chance.. It becomes very important to develop suitable preclinical in vitro and in vivo models to predict drug action.
- During drug screening, studies are performed to define the pharmacologic profile of the drug at the molecular, cellular, system, organ, and organism levels.
- At the molecular level the drug would be tested for receptor binding activity to target recptors, metabolizing enzymes etc.
- At the cellular level, the drug would be studied to determine whether it stimulates or inhibits the cellular function.
- At the organ level the pharmacologic activity and selectivity for the organ studied would be studied. Isolated organ studies esp. smooth muscles and other in vitro preparations can be used.
- Whole animal studies are generally necessary to determine the effect of the drug on organ systems and disease models. Cardiovascular and renal function studies of all new drugs are generally first performed in normal animals.
- Where appropriate, studies on disease models would be performed. Evidence would be collected on duration of action and efficacy following oral and parenteral administration.
- If the agent possessed useful activity, it would be further studied for possible adverse effects on other major organ systems, including the respiratory, gastrointestinal, endocrine, and central nervous systems.
- These studies might suggest the need for further chemical modification to achieve more desirable pharmacokinetic or pharmacodynamic properties. For example, oral administration studies might show that the drug was poorly absorbed or rapidly metabolized in the liver; modification to improve bioavailability might be indicated.
- If the drug was to be administered long-term, an assessment of tolerance development would be made. For drugs related to or having mechanisms of action similar to those known to cause physical dependence, abuse potential would also be studied. For each major action found, a pharmacologic mechanism would be sought.
- The outcome of this screening procedure is called a lead compound, i.e., a leading candidate for a successful new drug.
Preclinical safety and toxicity testing
- All drugs are toxic at some dose. To correctly define the toxicities of drugs and the therapeutic index comparing benefits and risks of a new drug is an essential part of the drug development.
- Most drug candidates fail to reach the market, due to the limitations of toxicity.
- Lead drugs that survive the initial screening and profiling procedures must be carefully evaluated for potential risks before and during clinical testing.
- Although no chemical can be certified as completely “safe” (free of risk), the objective is to estimate the risk associated with exposure to the drug candidate and to consider this in the context of therapeutic needs and duration of likely drug use.
- Toxicity testing is time-consuming and expensive. Two to six years may be required to collect and analyze data on toxicity and estimates of therapeutic index before the drug can be considered ready for testing in humans.
- Large numbers of animals may be needed to obtain valid preclinical data. Owing to the situation arising out of concerns of animal usage, animal testing has become a public issue and some segments of the public attempt to halt all animal testing in the unfounded belief that it has become unnecessary.
- Extrapolations of therapeutic index and toxicity data from animals to humans are reasonably predictive for many but not for all toxicities.
Clinical trials
In each of the three formal phases of clinical trials, volunteers or patients must be informed of the investigational status of the drug as well as the possible risks and must be allowed to decline or to consent to participate and receive the drug.
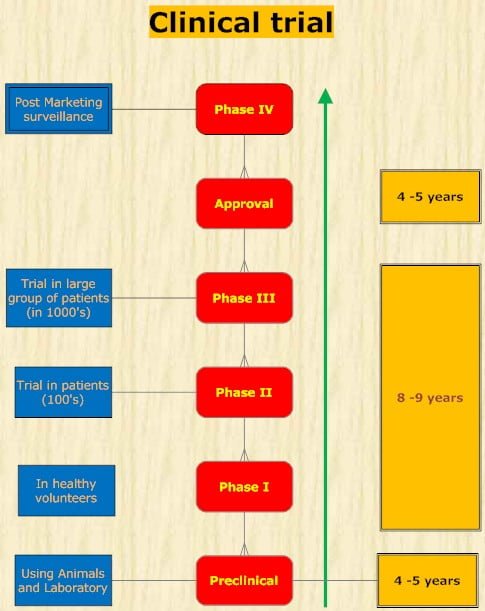
- In phase 1, the effects of the drug as a function of dosage are established in a small number (25-50) of healthy volunteers. If the drug is expected to have significant toxicity, as is often the case in cancer and AIDS therapy, volunteer patients with the disease are used in phase 1 rather than normal volunteers.
- Phase 1 trials are done to determine whether humans and animals show significantly different responses to the drug and to establish the probable limits of the safe clinical dosage range.
- These trials are nonblind or “open”; that is, both the investigators and the subjects know what is being given. Many predictable toxicities are detected in this phase.
- Pharmacokinetic measurements of absorption, half-life, and metabolism are often done in phase 1.
- Phase 1 studies are usually performed in research centers by specially trained clinical pharmacologists.
- In phase 2, the drug is studied in patients with the target disease to determine its efficacy.
- A modest number of patients (100-200) are studied in detail. A single-blind design is often used, with an inert placebo medication and an established active drug (positive control) in addition to the investigational agent.
- Phase 2 trials are usually done in special clinical centers (eg, university hospitals).
- A broader range of toxicities may be detected in this phase.
- In phase 3, the drug is evaluated in much larger numbers of patients with the target disease-sometimes thousands-to further establish safety and efficacy.
- Using information gathered in phases 1 and 2, phase 3 trials are designed to minimize errors caused by placebo effects, variable course of the disease, etc. Therefore, double-blind and crossover techniques are frequently used.
- Phase 3 trials are usually performed in settings similar to those anticipated for the ultimate use of the drug. The investigators are usually specialists in the disease being treated.
- Certain toxic effects, especially those caused by immunologic processes, may become apparent only in phase III.
- Phase 4 begins once approval to market a drug has been obtained.
- This constitutes monitoring the safety of the new drug under actual conditions of use in large numbers of patients.
- The careful and complete reporting of toxicity by physicians after marketing can reveal some side effects may after chronic dosing.
- Several hundred thousand patients may have to be exposed before the first case is observed of a toxicity that occurs with an average incidence of 1 in 10,000.
- Phase 4 has no fixed duration
Bioprospecting
- Refers to the search for useful applications, process or product with commercial value in the biosphere.
- It is the manipulation of nature’s bounty to find newer substances with economic importance.
- Many of the drugs in vogue today have come from traditional knowledge on the use of the plants in various biodiversity regions.
- After the discovery of penicillin, a systematic search of soil bacteria and microbes have led to the discovery of large groups of antibiotics, which are very successfully employed in the treatment of many bacterial infections in man and animals.
- Scientists are on a constant search into the deepest and farthest corners of the earth for a new product to be used as a drug for many diseases.
- Bioprospecting has its own issues and concerns. Often it leads to many new drug discoveries and treatment of diseases but the indigenous people who were instrumental are left to languish with no support and credit.
- Successful bioprospecting requires the development of a sustainable model in which a benefit sharing system is evolved among industries, ecosystem and the projected communities.
- Cure for many a dreaded diseases must be hidden in the remotest forests, deep oceans and highest mountains and bioprospecting is an instrument to uncover such new knowledge.
- When performed in a large-scale operation, the effort is referred to as mass bioprospecting.
- Experiences from the mass bioprospecting efforts demonstrate that mass bioprospecting is a complex process, involving expertise from diverse areas of human endeavors, what is important is the recognition of issues on genetic access, prior informed consent, intellectual property and the sharing of benefits that may arise as a result of the effort.
- Future mass bioprospecting endeavors must take heed of the lessons learned from past and present experiences in the planning for a successful mass bioprospecting venture.
Assay of drugs
Assay of drugs refers to the quantitative estimation of a drug / its active component in a formulation.
- It is carried out for the following purposes:
- To test the purity of a drug preparation.
- To compare the potency of a drug with that of a standard.
- To assess the efficiency of preparation while purifying the drug from a mixture / natural product.
Assays are of different types described below-
- Chemical assay– In this the concentration of drug is determined by chemical methods suah as spectrophotometry, fluorometry, HPLC or mass spectroscopy. These methods use one of the physiochemical properties of the compounds such as absorbance, fluorescence, polarity, charge to mass ratio etc. These are the most commonly used methods.
- Bioassay– In this the concentration of biologically active component in a unit quantity of the test drug is compared to the response produced by the standard or reference drug. Bioassays are more cumbersome but less accurate when compared to chemical assays. Eg: assay of histamine in isolated guinea pig ileum, assay of acetylcholine in frog rectus abdominis muscle and assay of antibiotics using bacteria.
- Immunoassay– this is commonly used for the assay of hormones. The method depends on the binding between the radiolabelled hormone and the corresponding antibody. The method is highly sensitive to detect minute quantities of the hormones in body fluids.