TABLE OF CONTENTS
Absorption of drugs
Absorption is the movement of drug from the site of administration to circulation. Often the entire quantity of administered drug does not reach the systemic circulation. Only the fraction of drug that was absorbed is available for action.
During the process of absorption, the quantity of drug absorbed and also the rate of drug absorbed determine the drug action.
In all routes of administration, except with intravenous administration, the drugs have to cross the biological membranes for entering the systemic circulation and hence are governed by factors affecting passage of drugs across membranes.
Absorption of drugs from the gastrointestinal tract
Before a drug can be absorbed, it must dissolve in the aqueous contents of the gut. Thus, the actual amount of the drug present in a dose is only one of the factors that will affect the amount of drug actually absorbed or available.
there is some factors affecting absorption of drug through GI route, include-
- Molecular size of the drug and its concentration
- Degree of ionization (depends on the pKa of the drug and pH of the medium)
- Lipid solubility of the neutral or nonionized form of the drug.
- Chemical or physical interaction with co-administered preparations and food constituents
- Pharmaceutical preparation and dosage form, especially their disintegration rate and dissolution rate
- Gastric motility and secretion as well as gastric emptying
- Intestinal motility and secretion as well as intestinal transit time
- Fluid volume within the gastrointestinal tract
- Osmolality of the intestinal contents
- Intestinal blood and lymph flow
- Disruption of the functional and structural integrity of the gastric and intestinal epithelium
- Drug biotransformation within the intestinal lumen by microflora or within the mucosa by host enzymes
- Volume and surface area of the absorbing surface. Stomach has a relatively small surface area compared to the duodenum. Hence absorption is more in the duodenum.
- Presence of food in the stomach. Normally in full stomach there is a delay in absorption because the drug gets diluted in the stomach contents.
Role of ionization and lipid solubility in drug absorption
Many drugs are weak electrolytes ie. they can ionize to a greater or lesser extent, according to environmental pH. Usually most molecules are present partly in the ionized and partly in the un-ionized state. The degree of ionization influences lipid solubility and, in turn, their absorption, distribution and elimination.
Note
Ionized / hydrophilic nature drugs absorbs very slowly and also not pass blood brain barrier rather than non-ionized / lipophilic drug.
The degree to which these drugs are lipid soluble (nonionized, the form in which drugs are able to cross membranes) is determined by their pKa and the pH of the medium containing the drug.
pKa of a drug is the pH at which 50% of the drug is ionized and 50% is non-ionized.
In monogastric animals with a low stomach pH, weak acids such as aspirin with a pKa of 3.5 tend to be better absorbed from the stomach than the weak bases because of the acidic conditions.
Weak bases are poorly absorbed from the stomach because they exist mostly in the ionized state in the acidic environment of the stomach . Weak bases are better absorbed from the small intestine where the environmental pH is more alkaline.
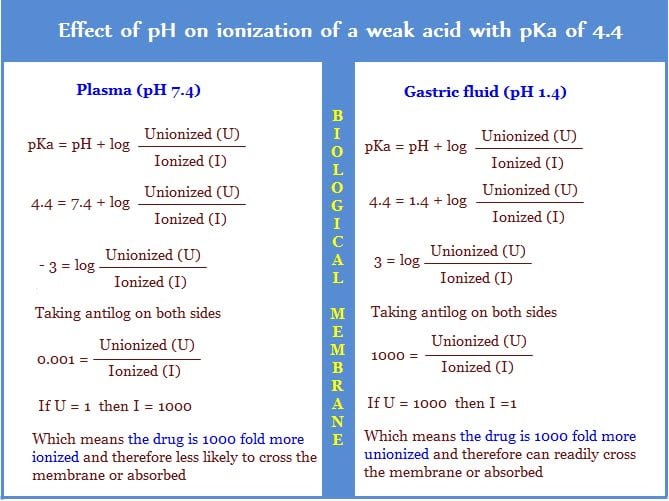
Henderson and Hasselbalch equation
Henderson Hasselbalch equation is used to calculate the percent of a drug that exists in ionized form or to determine the concentration of a drug across the biologic membrane.

Given this formula, if a drug is having a pKa of 4.4, and given the pH of plasma and gastric mucosa are 7.4 and 4.4 respectively, then
Absorption of drugs after parenteral administration
Drugs injected intramuscularly or subcutaneously may readily diffuse through tissue fluid and reach a capillary to be absorbed. Anything that interferes with diffusion of the drug from the site of administration or alters the blood flow to the injection site can delay absorption of the drug. The major factor that determines the absorption is the blood flow to the muscle.
Aqueous solutions of drugs are usually absorbed from intramuscular injection site within 10-30 minutes provided the blood flow is unimpaired. Faster or slower absorption is possible, depending on the concentration and lipid solubility of the drug, vascularity of the site, volume of injection, the osmolality of the solution and other pharmaceutical factors.
Absorption of drugs from subcutaneous tissues is influenced by the same factors that determine the rate of absorption from intramuscular sites. Some drugs are absorbed as rapidly from subcutaneous tissues as from muscles, although absorption from injection sites in subcutaneous fat is always significantly delayed.
Increasing the blood supply to the injection site by heating, massage or exercise hastens the rate of absorption. Spreading and absorption of a large fluid volume, which has been injected subcutaneously may be facilitated by including hyaluronidase in the solution.
Absorption of drugs after topical administration
Drugs may be absorbed through the skin following topical application. The intact skin allows the passage of small lipophilic substances, but efficiently retards the diffusion of water soluble molecules in most cases.
Highly lipid soluble preparations may be absorbed in considerable proportions encouraging the use of the topical route especially in veterinary practice. Pour on preparations of anthelmintics is a typical example.
Lipid insoluble drugs generally penetrate the skin slowly in comparison with their rates of absorption through the other body membranes. Absorption of drugs through the skin may be enhanced by inunction or more rarely by iontophoresis if the compound is ionized. Certain solvents like dmethylsulfoxide may facilitate the penetration of drugs through the skin.
Damaged, inflamed or hyperemic skin allows many drugs to penetrate the dermal barrier much more readily. The same principles that govern the absorption of drugs through the skin also apply to the application of topical preparations on the epithelial surfaces.
Many drugs traverse the cornea at rates that are related to their degree of ionization and lipid solubility. Thus organic bases such as atropine, ephedrine and pilocarpine often penetrate quite readily, whereas the highly polar aminoglycoside antibiotics generally penetrate cornea poorly.
Absorption of drugs after inhalation
The volatile and gaseous anaesthetics are the most important group of drugs administered by inhalation. These substances enter the circulation by diffusion across the alveolar membranes. Since they all have relatively high lipid water partition coefficients and generally are rather small molecules, they equilibrate practically instantaneously with the blood in the alveolar capillaries.
Particles contained in aerosols may be deposited, depending on the size of the droplets, on the mucosal surface of the bronchi or bronchioles, or even in the alveoli.